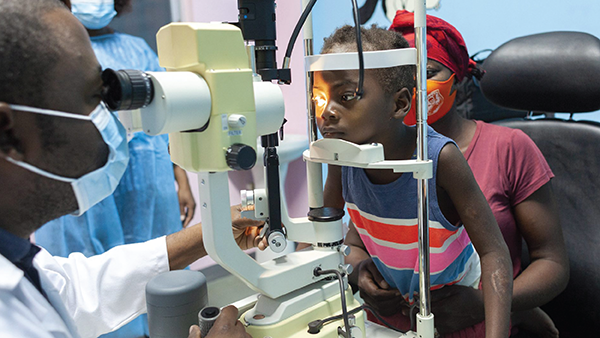
Redressing Representation
New JAMA Ophthalmology study highlights under-representation of racial and ethnic minorities in pediatric clinical trials
The under-representation of racial and ethnic minorities in clinical studies has been well-documented in existing literature; as recently as 2022, a report funded by the National Institutes of Health highlighted the continued lack of representation of these groups, existing “across numerous fields of medical research,” including cardiovascular, oncology, surgical trials, and ophthalmology (1).
The same issue affects pediatric ophthalmology, with a new JAMA Ophthalmology paper, “Race, Ethnicity, and Sex in Pediatric Eye Disease Investigator Group Clinical Studies,” revealing “a long-standing under-representation of Black, Asian, and Hispanic patients in PEDIG [Pediatric Eye Disease Investigator Group] studies,” says author Muhammad Z. Chauhan.
Chauhan and his team conducted a cross-section study of 41 completed PEDIG clinical trials involving 11,658 participants between 1997 to 2022. The researchers were primarily concerned with looking at how these trials reflected racial, ethnic, and gender representation in pediatric ophthalmology, and whether the trial participants accurately reflected the US pediatric population as reported in the 2010 US Census.
Using a 1-sample Wilcoxon rank test to compare results, the authors revealed that white participants were significantly over-represented in these clinical studies, whereas their Black, Hispanic, and Asian counterparts were underrepresented.
Specifically, the enrollment-census difference (ECD) – “defined as the difference between groups’ median enrollment percentage and percentage representation in the 2010 US Census” (2) – showed a 19 percentage over-representation of white participants, with Hispanic, Black, and Asian participants being underrepresented by 9 percent, 7 percent, and 3 percent respectively.
Given these results, the study recommends implementing changes in trial recruitment practices (e.g., “identifying and improving existing biases in recruiting” and incorporating enrollment limits based on disease prevalence).
Meanwhile, several interventions in PEDIG have already been implemented to better engage under-represented pediatric populations, notes co-author Abdelrahman M. Elhusseiny. “These include expanding access to enrollment materials in languages other than English, partnering with trusted healthcare providers who are well-established within communities and can advocate for PEDIG, and the creation of a dedicated committee to actively promote equity, diversity, and inclusion in study enrollment.” These initiatives have already begun to yield measurable improvements, notes Chauhan. “Notably, within just a short span of two years, we saw a marked increase in Hispanic patient enrollment, highlighting the positive impact of these efforts.”
The researchers believe there is still much work to be done within the space, however. “Advocating for expanded ophthalmology services in rural and socioeconomically underserved regions is essential,” adds co-author Qais A. Dihan. In addition, Dihan says that the socioeconomic barriers preventing families from participating in these clinical trials – such as transportation and flexibility of scheduling – also need to be addressed if we are to “resolve underlying issues that contribute to, and perpetuate, a cycle of inequitable care in underserved regions.”
The New Optometrist Newsletter
Permission Statement
By opting-in, you agree to receive email communications from The New Optometrist. You will stay up-to-date with optometry content, news, events and sponsors information.
You can view our privacy policy here
Most Popular
Sign up to The New Optometrist Updates
Permission Statement
By opting-in, you agree to receive email communications from The New Optometrist. You will stay up-to-date with optometry content, news, events and sponsors information.
You can view our privacy policy here
Sign up to The New Optometrist Updates
Permission Statement
By opting-in, you agree to receive email communications from The New Optometrist. You will stay up-to-date with optometry content, news, events and sponsors information.
You can view our privacy policy here