You are viewing 1 of your 3 articles before login/registration is required
Treating DED Before Cataract Surgery
Studies show about 80 percent of cataract patients present with dry eye disease, which can skew testing and adversely affect outcomes. Addressing DED before cataract surgery can prevent three potential problems, writes William B. Trattler
Though estimates of dry eye disease (DED) prevalence vary widely, it is well documented that the risk of DED increases with age (1). No doubt that’s one reason that DED is so common in our cataract patients. In a 2017 study, my colleagues and I found that most patients presenting for cataract surgery had no symptomatic complaints, yet 62.9 percent had a TBUT ≤5 seconds and 77 percent had corneal staining (2). A study the following year showed that about half of patients had DED symptoms, but a close look at asymptomatic patients revealed that 85 percent had abnormal results for at least one DED test (3).
With DED affecting so many cataract patients, treating the condition before surgery –which ensures that patients have optimal postoperative vision and comfort – has become part of the standard of care for both surgeons and referring optometrists.
We’re accustomed to treating the discomfort associated with DED, but that’s only part of the picture. DED affects cataract patients’ vision and preoperative measurements, and its presence preoperatively virtually ensures patients will continue to battle the problem postoperatively. By addressing DED before cataract surgery, we head off three potential problems:
- A secondary cause of blurry vision
Most of my cataract patients with DED don’t complain of discomfort, but they do have visual symptoms. DED is often a contributing factor to their blurry vision, which they assume is entirely caused by cataracts. When I ask about their vision, some cataract patients share that their blurry vision fluctuates, getting worse throughout the day or improving after they blink. I see some severe cases where DED presents as a greater vision problem than the cataracts, and once we treat their dry eye, the patient can see more clearly and can hold off on cataract surgery. - Impact on measurements and IOL calculations
DED impacts the accuracy of biometry and topography, which in turn affects our IOL calculations. This impact applies to both monofocal and multifocal lenses, but the most marked differences are apparent in astigmatism measurements. When I treat patients for DED and bring them back for new measurements, it’s common to find a significant difference that would have led me to choose an IOL that would produce unsatisfactory results. For example, sometimes astigmatism is not obvious, but we can see it clearly after treating DED; in other cases, we see less astigmatism after DED treatment. By treating DED and bringing patients back to repeat their measurements, we know that we’re making decisions based on accurate readings. - Postoperative exacerbation of DED
Surgery and postoperative care can exacerbate DED – both from the potential impact of small incisions and from using preserved eye drop medications for 4–6 weeks postoperatively. As a result, even patients with no preoperative pain or irritation can develop discomfort as nerves become sensitized after surgery.
Whether we fail to address blurriness caused by DED, choose the wrong IOL based on inaccurate measurements, or allow a patient to progress to DED discomfort postoperatively, patients will be dissatisfied and may even blame DED on the cataract surgery – even if the problem was there all along.
Pre- and post-op DED treatment
When patients are referred for cataract surgery, they want surgery as soon as possible. They’re looking forward to getting back to their lives with better vision. I explain that, like most cataract patients, they have a second condition called DED, and we need to treat it now to get the best results from surgery.
I use fast-acting therapies for DED, so patients are happy and treatment rarely delays their surgery. One new product that I have had a very positive experience with is Ivizia (Thea), which is a very comfortable multi-dose preservative-free artificial tear. With lubricating povidone as the active ingredient, it also offers the combination of hyaluronic acid (HA) and trehalose, which is proven more effective than HA alone at maintaining an effective tear film and improving DED symptoms (4–7).
Because many cataract patients have no discomfort from their DED, it’s important to give them a 3-4 times per day schedule for using artificial tears, rather than telling them to use tears as needed. I often use a steroid, such as loteprednol (Eysuvis, Kala; Lotemax, Bausch & Lomb) ), or prednisilone acetate 1 percent twice daily for 2–3 weeks to quickly improve inflammation and staining.
For more advanced DED, I prescribe an immunomodulator (Cequa, Sun Pharma; Restasis, Allergan; Xiidra, Novartis). I also recommend hypochlorous acid spray eyelid cleanser (Avenova, Novabay Pharma) when there appears to be an eyelid hygiene component. Artificial tears, hygiene and immunomodulators are continued after surgery. I prefer preservative-free artificial tears for all patients with DED, but they are essential for DED treatment in the postoperative period when patients are using multiple preserved topical drops every day.
Case study: Grade 3 cataracts and significant DED
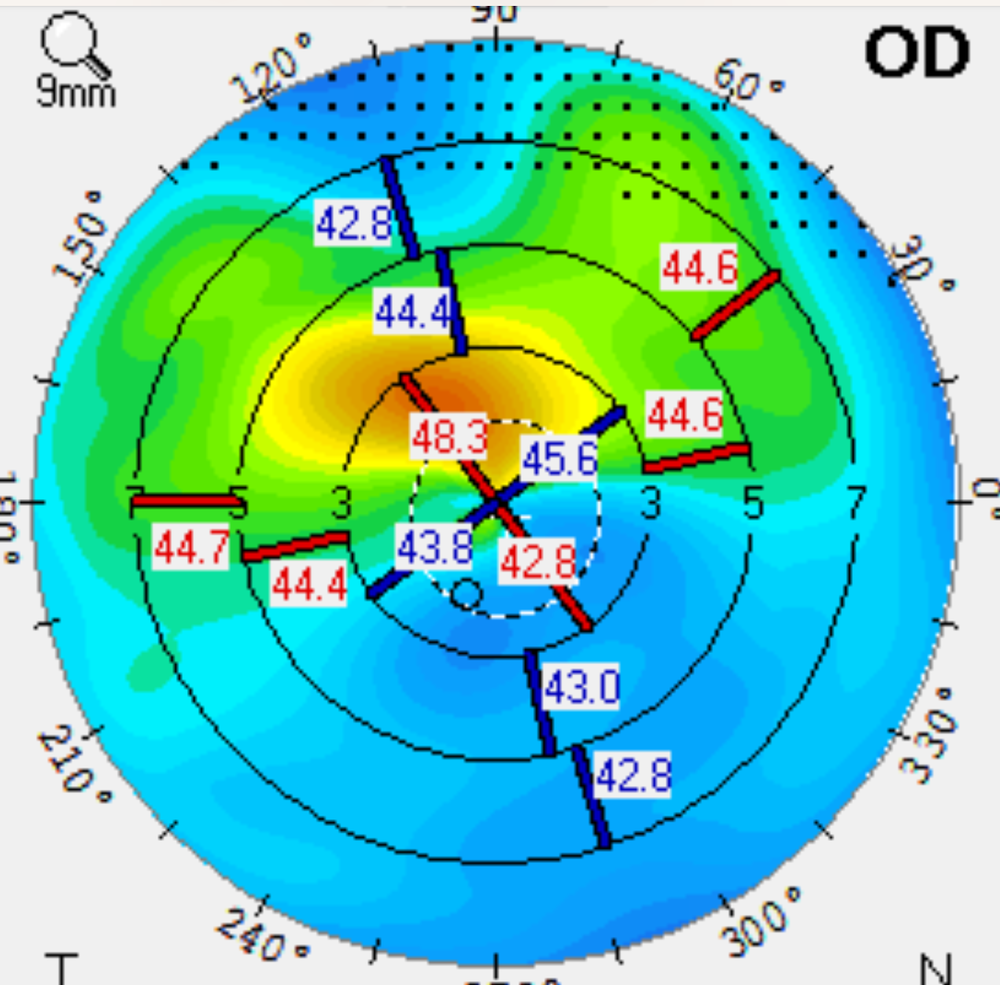
A 70-year-old man with 2+ nuclear sclerotic (NS) cataracts was referred for surgery, with visual goals to read and drive comfortably without glasses. He complained of blurry, fluctuating vision, but he wasn’t experiencing discomfort. His best-corrected vision was 20/50, and he had a visually significant cataract present. Three automated tests to measure preoperative astigmatism (Pentacam, Cassini, and IOL Master 700) showed variation, rather than the agreement we typically see with healthy corneas. The patient had central corneal staining and a tear breakup time of five seconds in both eyes. I noted some eyelid crust as well.
I started the patient on preservative-free Ivizia artificial tears four times per day for lubrication and hydration and a two-week course of prednisolone acetate to reduce inflammation. Both of these drops come in multi-dose bottles that are easy to use for all patients, including seniors. I also had the patient use Avenova eyelid hygiene to help reduce the bacteria load prior to surgery, as well as reduce the amount of bacteria secreting lipases that make the meibomian gland secretions extra thick.
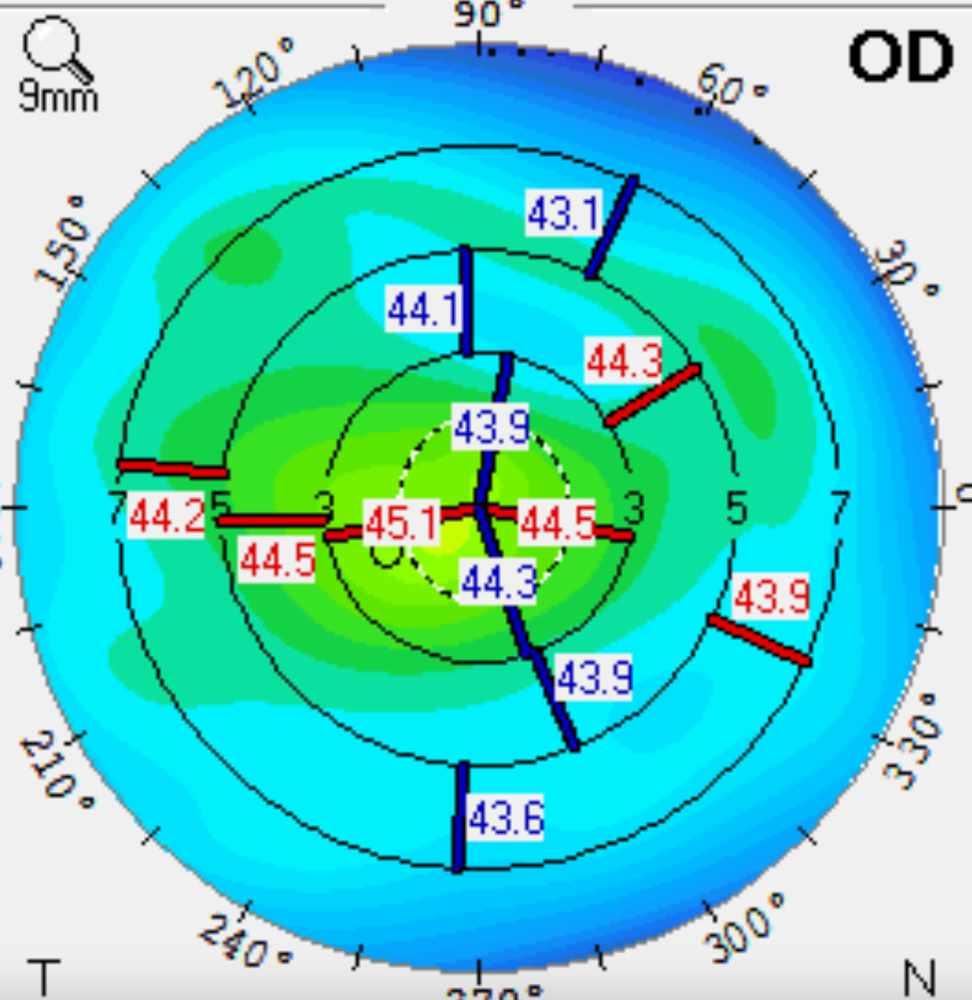
When the patient came back, his topographic maps had a more symmetrical appearance. The staining was resolved as well. Tear breakup time was 12 seconds oculus. Before DED therapy, the topography showed significant irregular astigmatism, making the patient ineligible for a trifocal or EDOF IOL (see Figure 1). Following treatment with Ivizia, prednisolone acetate BID, and Avenova BID for three weeks, there was significant improvement in the corneal shape (see Figure 2).
To meet the patient’s visual goals for reading and driving, we chose a multifocal IOL. The patient was very happy with his postoperative visual acuity. His eyes remained comfortable, and he continued using his Ivizia artificial tears in the morning. Because DED is chronic and progressive, I recommended that he also start Cequa BID.
Because patients like this are very much the norm, I talk to my referring optometrists about how they can help the process along. They are very good at catching symptomatic DED, and once they know that many cataract patients have asymptomatic DED, they can detect the problem and start DED therapy earlier in the process of cataract formation, so the ocular surface is healthy before patients need surgery.
References
- CS de Paiva CS, “Effects of Aging in Dry Eye,” Int Ophthalmol Clin, 57, 47 (2017). DOI:10.1097/IIO.0000000000000170
- WB Trattler et al., “The Prospective Health Assessment of Cataract Patients' Ocular Surface (PHACO) study: the effect of dry eye,” Clin Ophthalmol, 11, 1423 (2017). DOI:10.2147/OPTH.S120159
- PK Gupta et al., “Prevalence of ocular surface dysfunction in patients presenting for cataract surgery evaluation,” J Cataract Refract Surg, 44, 1090 (2018).. DOI:10.1016/j.jcrs.2018.06.026
- D Schmidl et al., “Tear film thickness after treatment with artificial tears in patients with moderate dry eye disease, Cornea, 34, 421 (2015).
- K Fondi et al., “Effect of Hyaluronic Acid/Trehalose in Two Different Formulations on Signs and Symptoms in Patients with Moderate to Severe Dry Eye Disease,” J Ophthalmol (2018). DOI: 10.1155/2018/4691417. PMID: 30155282
- F Chiambaretta et al., “A randomized, controlled study of the efficacy and safety of a new eyedrop formulation for moderate to severe dry eye syndrome,” Eur J Ophthalmol, 27, 1 (2017).
- R Mencucci et al., “Hyaluronic Acid/Trehalose Ophthalmic Solution in Reducing Post-Cataract Surgery Dry Eye Signs and Symptoms: A Prospective, Interventional, Randomized, Open-Label Study,” J Clin Med, 10, 4699 (2021).
The New Optometrist Newsletter
Permission Statement
By opting-in, you agree to receive email communications from The New Optometrist. You will stay up-to-date with optometry content, news, events and sponsors information.
You can view our privacy policy here
Most Popular
Sign up to The New Optometrist Updates
Permission Statement
By opting-in, you agree to receive email communications from The New Optometrist. You will stay up-to-date with optometry content, news, events and sponsors information.
You can view our privacy policy here
Sign up to The New Optometrist Updates
Permission Statement
By opting-in, you agree to receive email communications from The New Optometrist. You will stay up-to-date with optometry content, news, events and sponsors information.
You can view our privacy policy here