You are viewing 1 of your 3 articles before login/registration is required
Advancing AI in Eye Care
Why expanding and diversifying retinal image datasets will help improve the effectiveness of deep learning-based tools
Artificial Intelligence (AI) is no longer the stuff of science fiction – it’s present all around us in everything from social media feeds to factories, helping to power our busy modern lives. It is used in many different healthcare settings, including monitoring patients with heart disease and improving the early diagnosis of cancer (1). But what exactly is AI? How does it learn? And how can it be used in eye care?
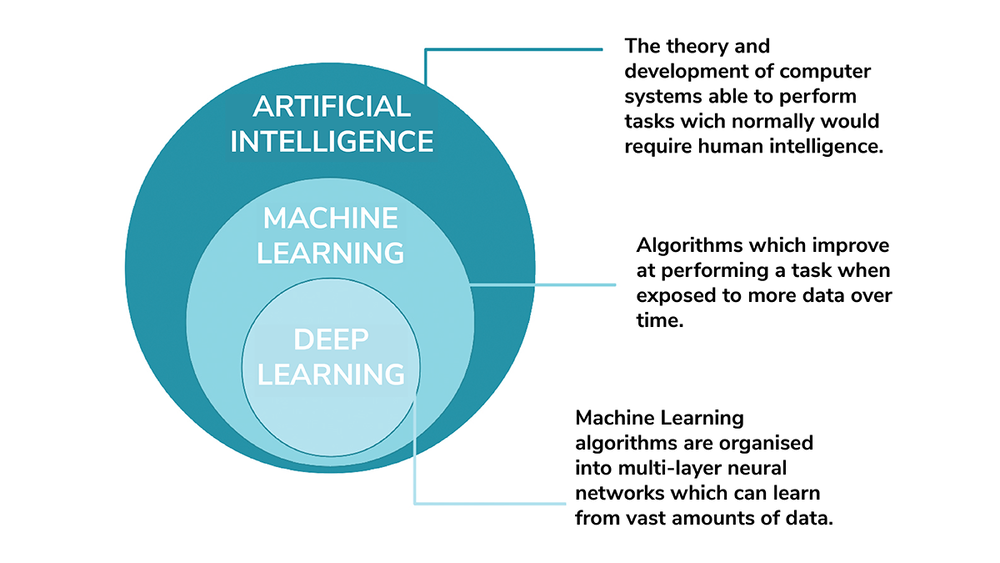
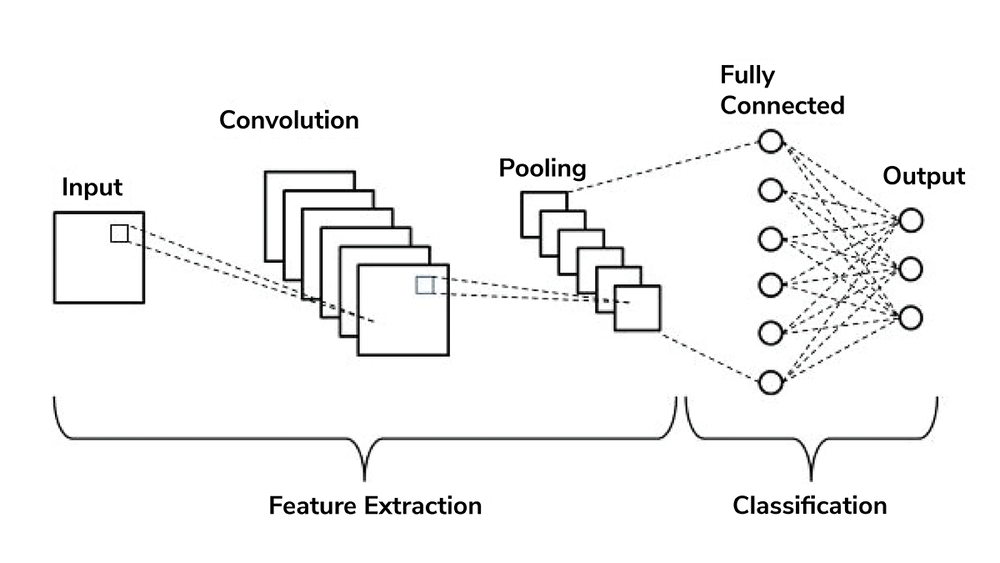
AI is an umbrella term covering the theory and development of computer systems able to perform tasks that would normally require human intelligence. Machine Learning (ML) is a subset of AI, consisting of algorithms that learn from data in order to make decisions. An ML system’s performance at a specific decision-making task improves as it is exposed to more data. Deep Learning (DL) is a subset of ML, which organizes ML algorithms into many layers to create a structure known as a convolutional neural network (CNN). CNNs use very large data sets to learn how best to perform a specific task. DL systems are what most people think of when they think about artificial intelligence.
Currently, DL systems can only learn how to perform very specific tasks. They cannot teach themselves how to perform tasks where their learning has not been facilitated – so there is no risk of developing a Terminator-style Skynet super intelligence (yet).
CNNs use pattern recognition to help them learn how to perform a specific task. If we wanted to train a CNN how to detect age-related macular degeneration (AMD), we would require a large data set of retinal images, including lots of images of AMD. The CNN would use pattern recognition to learn what features of the image are associated with AMD, and its accuracy at detecting AMD would improve as it was shown more and more retinal images. This method of learning allows the CNN to detect areas of importance in the image that may not have been seen by a human observer.
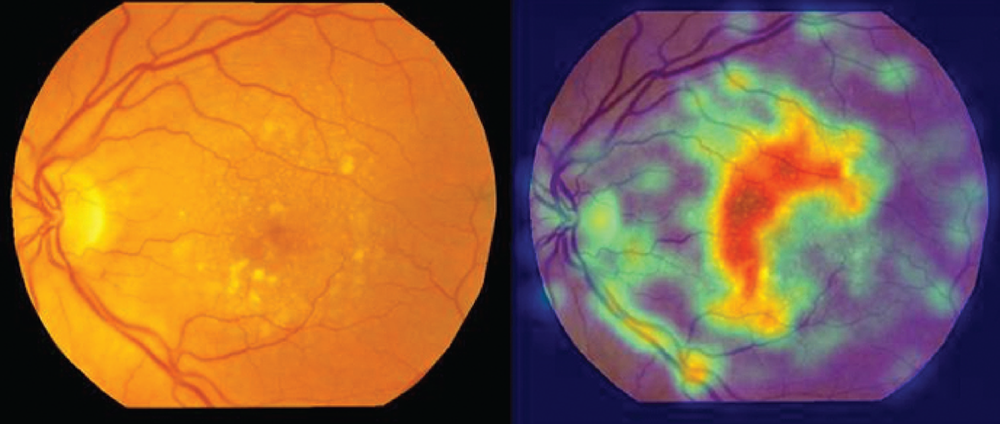
Researchers at the University of Edinburgh and the University of Dundee have found that the size, number, and distribution pattern of drusen in AMD may predict the progression of the disease (3). Quantifying drusen in this way would be impossible for an optometrist working in a busy clinic, but it takes a matter of milliseconds for a CNN (see Figure 3). In this way, deep learning AI tools could help optometrists in their clinical-decision making. But it’s important to remember that they are intended as decision-support assistants and not designed to replace the role of the optometrist in practice.
Deep learning is already being used in certain eye care settings; for example, AI tools have proved to be an accurate and cost-effective method of screening patients for diabetic retinopathy (4, 5). Such tools have the potential to greatly increase the capacity of the screening program, which is particularly valuable in an NHS that is trying to recover from the disruption caused by COVID-19.
However, a deep learning tool is only as good as the data that has been used to train and validate it. Many of the current retinal image data sets used to train deep learning tools have flaws, including poor quality images, limited ethnic diversity, or bias towards people with late-stage eye disease. Issues with poor quality or non-representative datasets can cause serious unintended consequences. Deep learning algorithms have been linked, for example, to racial bias when predicting crime hotspots, performing facial recognition on CCTV footage, or handling job applications (6).
The pressing need for a high-quality retinal image dataset that is representative of the population as a whole is one of the primary motivators of SCONe – the Scottish Collaborative Optometry-Ophthalmology Network e-research. The SCONe team, comprising optometrists, ophthalmologists, data scientists, and IT experts, is aiming to build a world-leading retinal image research resource in Scotland. Work on this has already begun by gathering retinal images captured in community optometry practices across the country and securely transferring them to the National Safe Haven (a secure storage facility run by Public Health Scotland).
Once in the National Safe Haven, the images are linked to NHS healthcare datasets and pseudonymized so that no personally-identifiable information is visible. The retinal images can then be used by approved researchers to discover new ways of detecting eye disease at an earlier stage, improve the prediction of progression of eye disease, and learn more about the links between the eyes and general health. Community optometrists in Scotland have been gathering retinal images for a decade or more, which will allow us to explore how the retina changes over time in both ocular and systemic disease. Moreover, any deep learning tools trained on the rich SCONe dataset will be more relevant and applicable to the patients we see every day in practice.
References
- K-H Yu et al., “Artificial intelligence in healthcare,” Nat. Biomedical Eng., 2, 719 (2018).
- VH Phung, EJ Rhee, “A High‐Accuracy Model Average Ensemble of Convolutional Neural Networks for Classification of Cloud Image Patches on Small Datasets,” Applied Sciences, 9, 4500 (2019).
- E Pead et al., “Automated detection of age-related macular degeneration in colour fundus photography: a systematic review,” Survey of Ophthalmology, 64, 498 (2019).
- GS Scotland et al., “Costs and consequences of automated algorithms versus manual grading for the detection of referable diabetic retinopathy,” Br J Ophthalmol., 94, 712 (2010).
- National Institute for Health and Care Excellence, “AI technologies for detecting diabetic retinopathy,” NICE, 2021.
- S Marsh, “UK police use of computer programs to predict crime sparks discimination warning,” The Guardian, February 3, 2019.
The New Optometrist Newsletter
Permission Statement
By opting-in, you agree to receive email communications from The New Optometrist. You will stay up-to-date with optometry content, news, events and sponsors information.
You can view our privacy policy here
Most Popular
Sign up to The New Optometrist Updates
Permission Statement
By opting-in, you agree to receive email communications from The New Optometrist. You will stay up-to-date with optometry content, news, events and sponsors information.
You can view our privacy policy here
Sign up to The New Optometrist Updates
Permission Statement
By opting-in, you agree to receive email communications from The New Optometrist. You will stay up-to-date with optometry content, news, events and sponsors information.
You can view our privacy policy here