You are viewing 1 of your 3 articles before login/registration is required
Expanding Access to Amniotic Membrane Transplantation
NuVision’s Omnigen and OmniLenz – taking amniotic membrane transplantation into clinics.
Amniotic membrane transplantation is known to support ocular healing, reduce the progression of inflammation, and reduce patient discomfort – all benefits that support the stabilization of the ocular surface. Amniotic membrane has been used as a tissue-transplant material since 1940 and has been popular in ophthalmology since the 1990s. Cryopreservation and freeze-drying are the traditional modes for preserving amniotic membranes; however, these methods can cause damage to the cellular structure. The potential of frozen amniotic membrane is also marred by cold-chain shipping and storage logistics and the requirement to defrost the tissue prior to use.
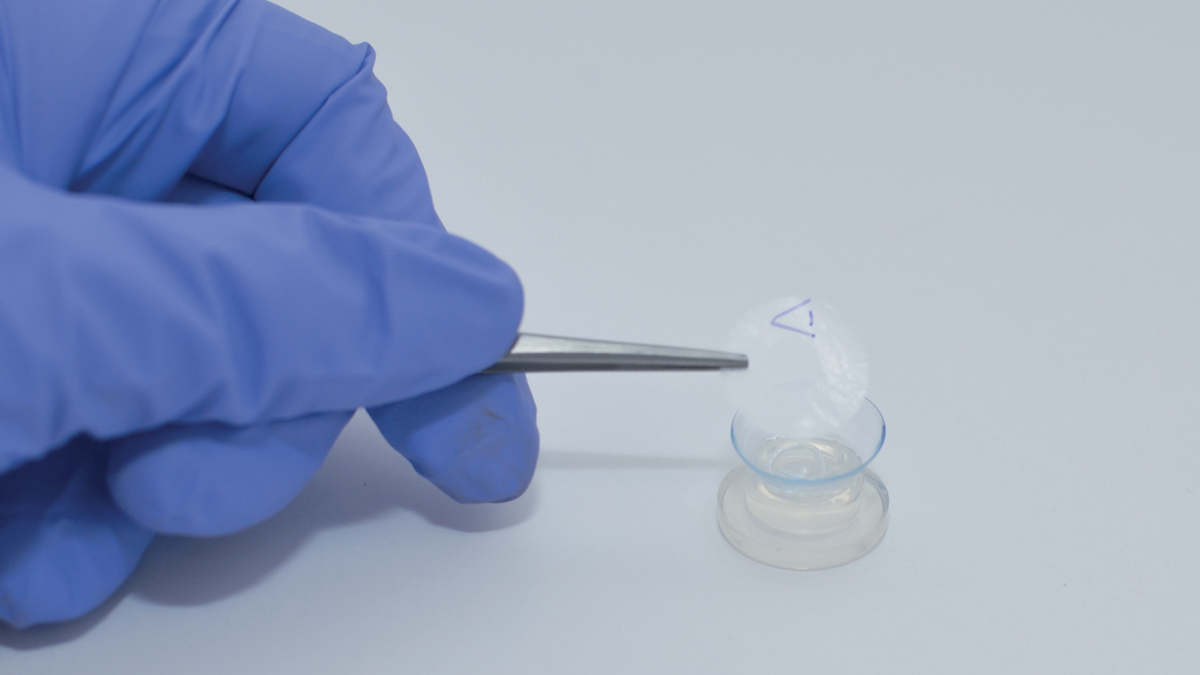
NuVision Biotherapies is a tissue-based therapy company that uses a gentle, low-temperature vacuum evaporation process to dry out the tissue without freezing it. The company collects the amniotic membrane, the innermost layer of the placental sac, from women undergoing elective cesarean section. Once the tissue is collected (with the women’s informed consent), it undergoes NuVision’s proprietary Tereo process, which creates Omnigen, an amniotic membrane that can be stored at 2–25°C and maintains the natural integrity of fresh amniotic membrane. In drying out the tissue without freezing it, the Tereo process prevents damage to its natural integrity and preserves its natural barrier function. Further, it yields a product that can easily be rehydrated from the native moisture at the ocular surface and can be stored at room temperature. Available in 10 sizes, Omnigen can be applied directly to the ocular surface, and does not require any preparation, making it easily available at the point of care.
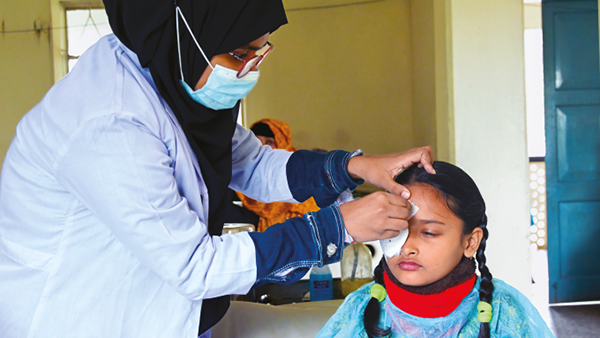
In conjunction with Menicon Ltd, NuVision has developed OmniLenz, a bandage contact lens that holds Omnigen at the ocular surface. Omnigen can be loaded into OmniLenz and placed onto the ocular surface in a simple four to six minute procedure. This allows for amniotic membrane transplantation to be delivered in a non-surgical setting, such as an outpatient clinic, opening up access to amniotic membrane transplantation to patients with moderate ocular surface disease. Additionally, OmniLenz can be administered by optometrists or trained nurse specialists.
Many chronic conditions, would benefit from amniotic membrane application, such as dry eye disease, as noted in the DEWS II report (1), but the surgical requirement often led to this being a later-stage therapy. Providing access to amniotic membrane through contact lens application, however, allows for the option of amniotic membrane transplantation earlier in the treatment pathway. In enabling the application of amniotic membrane in a non-surgical, outpatient setting, OmniLenz opens up this treatment to a large range of patients with moderate to severe ocular surface conditions and provides a more comfortable treatment process for the patient.
In a recent randomized-controlled trial in the treatment of moderate-to-severe dry eye disease (DED), patients were treated bilaterally with Omnigen-OmniLenz. The study results were presented at the Royal College of Ophthalmology Congress in May 2023 (2). Patients were treated for approximately one week with the amniotic membrane treatment, resulting in a significant reduction in ocular surface disease index (from 55.8 to 18.8), SANDE Frequency (68 to 40) and Severity (58 to 49). Patients saw a significant improvement in dry eye symptoms, as well as ocular surface health for at least three months. The treatment also promoted corneal nerve regeneration, positioning Omnigen-OmniLenz as an exciting short-term treatment for moderate-to-severe DED.
OmniLenz removes the burden of taking patients into surgery to apply amniotic membrane to the ocular surface. This means that patients can access the treatment earlier, there is a reduced cost of application, and other healthcare professionals are able to apply the therapy.
References
- TFOS DEWS II Management and Therapy Report, The Ocular Surface (2017).
- bit.ly/3Q0pfTd
The New Optometrist Newsletter
Permission Statement
By opting-in, you agree to receive email communications from The New Optometrist. You will stay up-to-date with optometry content, news, events and sponsors information.
You can view our privacy policy here
Most Popular
Sign up to The New Optometrist Updates
Permission Statement
By opting-in, you agree to receive email communications from The New Optometrist. You will stay up-to-date with optometry content, news, events and sponsors information.
You can view our privacy policy here
Sign up to The New Optometrist Updates
Permission Statement
By opting-in, you agree to receive email communications from The New Optometrist. You will stay up-to-date with optometry content, news, events and sponsors information.
You can view our privacy policy here